Models & Assays
Drug Self-administration
Self-administration is the gold-standard for assessing reinforcement. Self-administration studies assess voluntary consumption of a compound. Safety studies examine the ability of a novel compound to maintain operant responding for drug delivery compared to vehicle and a drug of abuse from the same class or therapeutic category. Efficacy studies evaluate whether a novel compound can reduce reinforcement of a known drug of abuse. Transpharmation offers oral and intravenous self-administration using a variety of schedules of reinforcement.
Aspects of self-administration: Acquisition, Dose-response, Progressive ratio (motivation), Reinstatement (model of relapse) and Drug pretreatment.
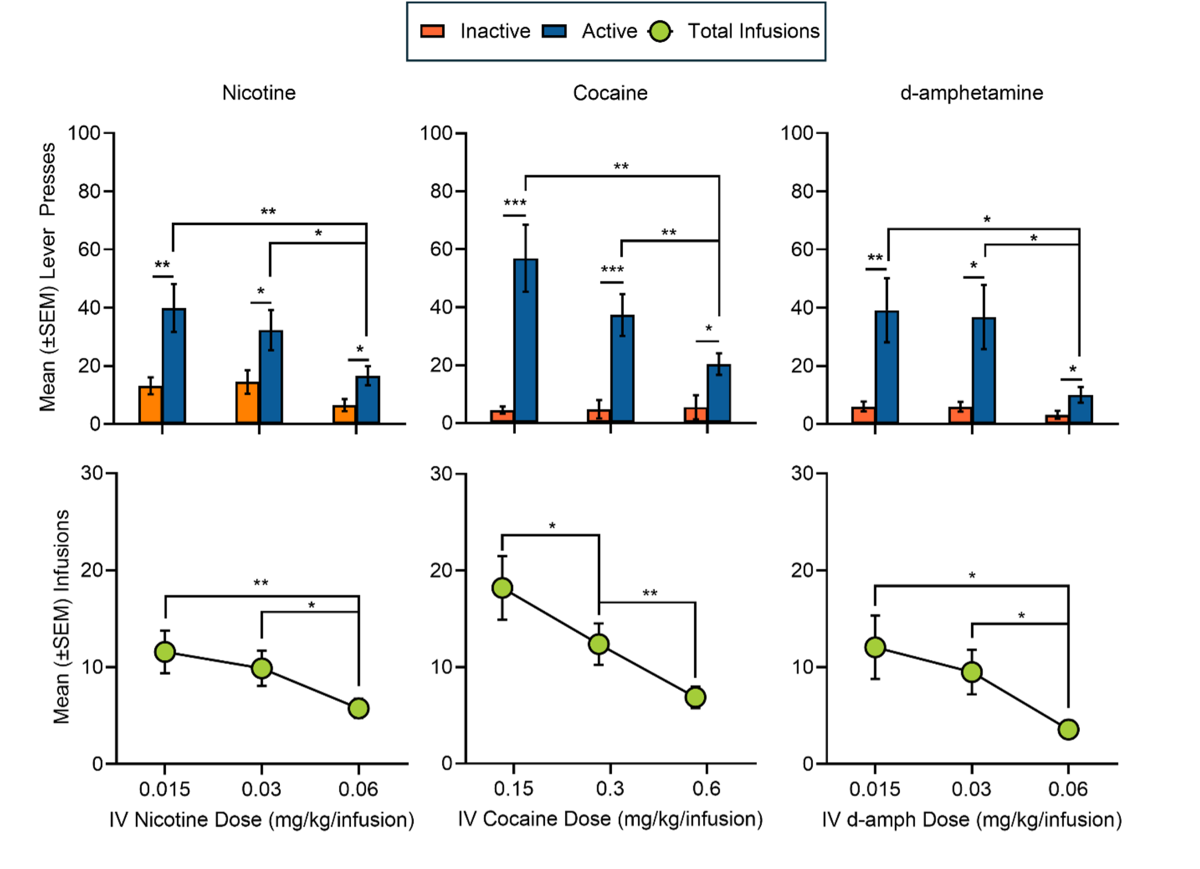
Self-administration: Dose Response
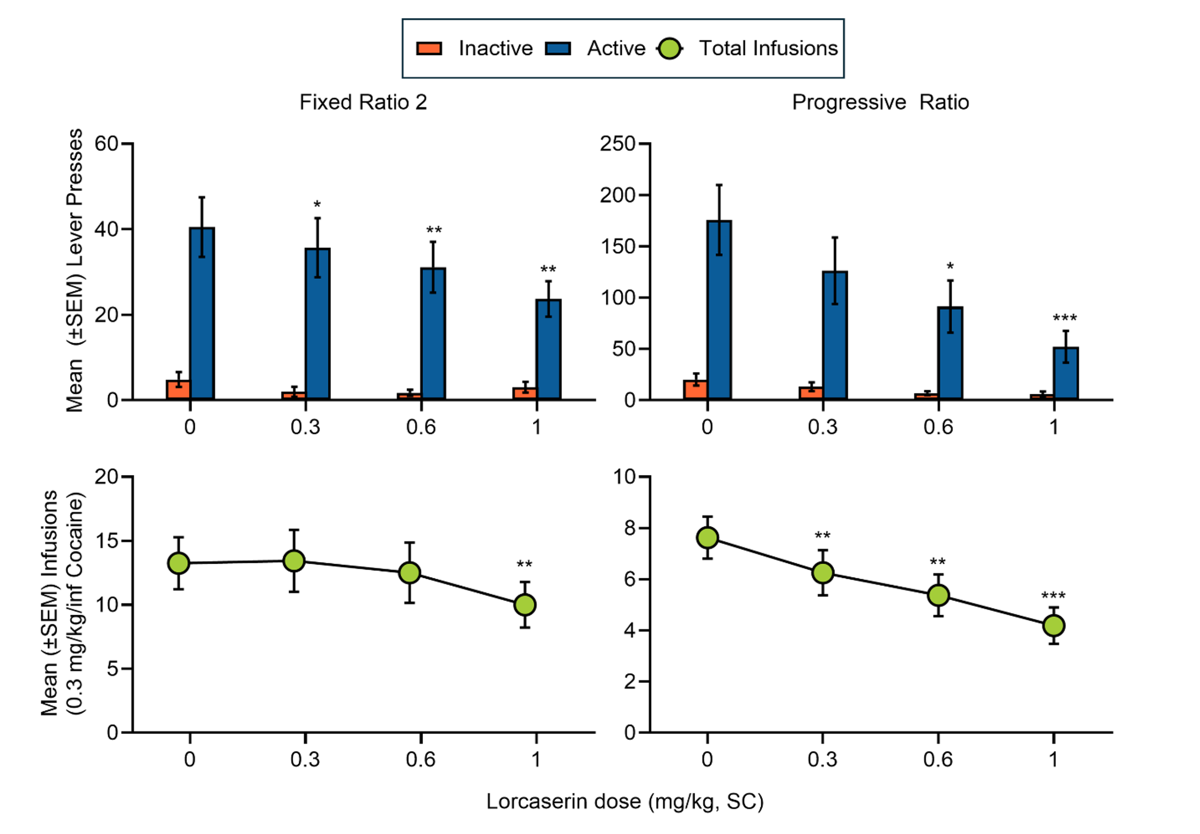
Self-administration: Drug Pretreatment
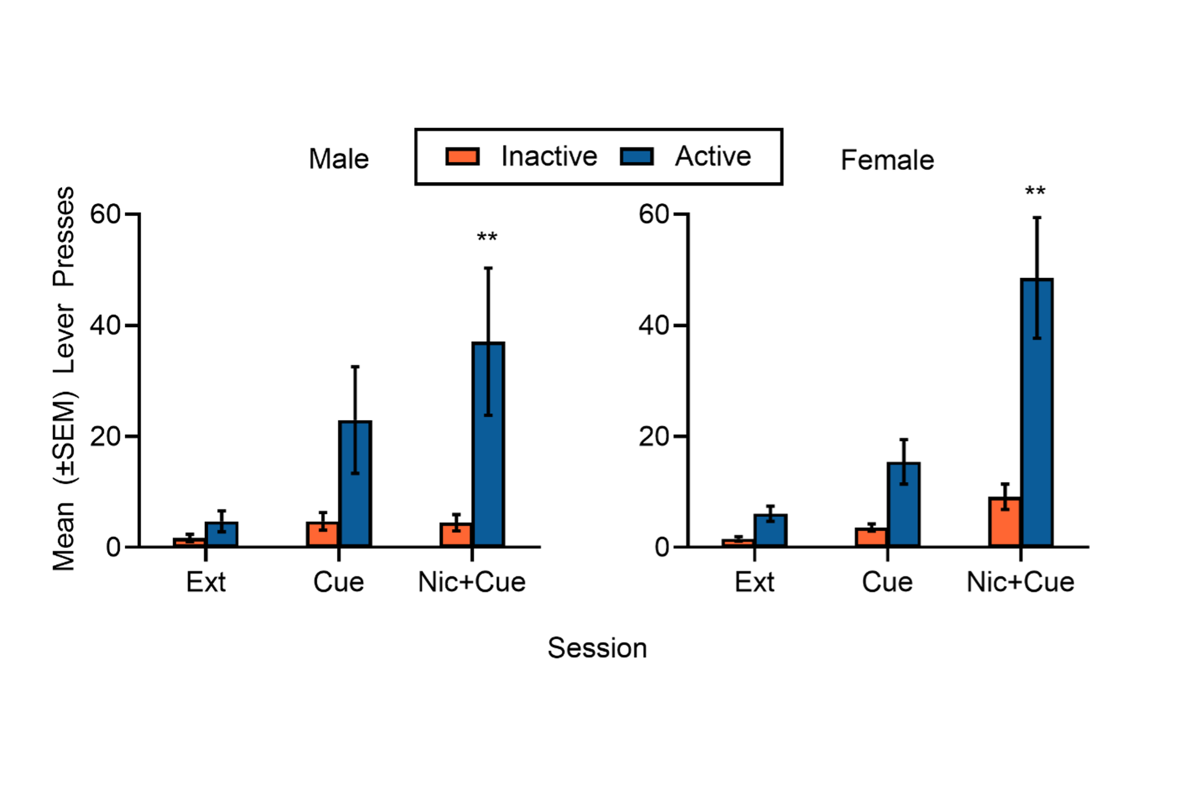
Self-administration: Reinstatement (model of relapse)